- solvation is the process of decomposition of a molecule due to its intermolecular interactions with other molecules
- solvent is any substance capable of solvation
- solute is any substance undergoing solvation
- solution is the mixture created by dissolving a solute in a solvent
Molecule movement in a solution
- molecules in a solution orient themselves so that the charged particles (or dipoles) attract one another
- polar solvents dissolve polar molecules
- the molecules of solvent attack the molecules of solute and rip them appart forming micelle-like structures around the ions of the compound
Describing mixtures
Mass percent/fraction $w$
- it is the ratio of the mass of the solute to the mass of the solution
$$w=\cfrac{m_i}{m}$$
Molarity $c$
- molarity represents the number of moles of the solute in a volume of the solution
- it can be used to describe solutions which are hard to weigh
- it is prefered in most cases
- the base unit is $mol\cdot{dm^{-3}}$
- it is usually written as just $M$
$$c=\cfrac{n_i}{V}$$
Molality $b$
- molality is the reation of the number of moles of the solute to the mass of the solvent
- it is widely used in biochemistry
- the base unit is $mol\cdot{kg^{-1}}$
- is is usually written as just $m$
$$b=\cfrac{n_i}{m_s}$$
Concentrating/Diluting a solution
- mass percent equilibrium
$$m_{i1}=m_{i2}$$ $$w_1m_1=w_2m_2$$
- molarity/molality equilibrium
$$n_{i1}=n_{i2}$$ $$c_1V_1=c_2V_2$$ $$b_1m_{s1}=b_2m_{s2}$$
Dissociation and reaction schemes
- dissociation is a chemical reaction in which a molecule is broken up into its individual parts
- chemical reactions can be represented in rection schemes
- rection scheme of a dissolution in water: $AB(s)\overset{H_2O}{\longrightarrow}Na^+(aq)+Cl^-(aq)$
- the state of the compounds can be represented using letters in brackets behind the formula
- $(s)$ - solid
- $(l)$ - liquid
- $(g)$ - gas
- $(aq)$ - aqeous solution
- the substance that causes the reaction to happen (reactant) is often placed above the arrow
- the substance that is produced during the reaction but is unimportant for further continuation of the reaction is often placed under the arrow
- when no more substance can be disolved, we call it the point of saturation
Electrolytes
- electrolyte is a substance the solution in a polar solvent of which conducts electricity
- oposite is nonelectrolyte
- strong electrolyte dissolves fully and easily in a polar solvent
- an example is sodium chloride $NaCl$
- they are formed by strong acids and strong bases
- dissolution of sodium chloride in water:
$$NaCl(s)\overset{H_2O}{\longrightarrow}Na^+(aq)+Cl^-(aq)$$
- weak electrolyte doesn’t dissolve properly or in its entirety in a polar solvent
- an example is sodium fluoride $NaF$
- they are formed by weak acid and strong base, strong acid and weak base or weak acid and weak base
- they alter the pH of the solution
- dissolution of sodium fluoride in water $$NaF(s)\overset{H_2O}{\longrightarrow}Na^+(aq)+HF(aq)+OH^-(aq)$$
Effects on solubility
- the amount of disolved salts rises with the rising temperature
- the increasing temeprature weakens the bonds in the solute, thus making it more susceptable to dissolution
- higher speeds of molecules of the solvent make it easier to break up crystal lattices
- the amount of disolved gas decreases with the rising temperature
- the higher speed of the molecules of the solute make it easier to break from the attractive forces of the solvent
Separation methods
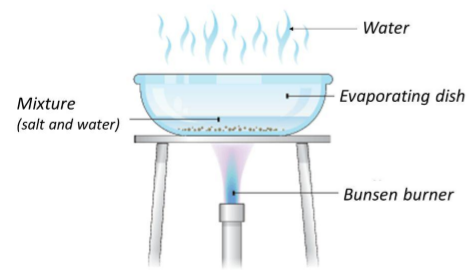
Evaporation
- a solution is heated up and the solvent is boiled of leaving the solute in a crystallizzed form
- should there be more solutes, the result will be the mix of them
- should the solute be thermically unstable, evaporation should not be used
- it is usually done under low pressure to lower the boiling point of the solvent
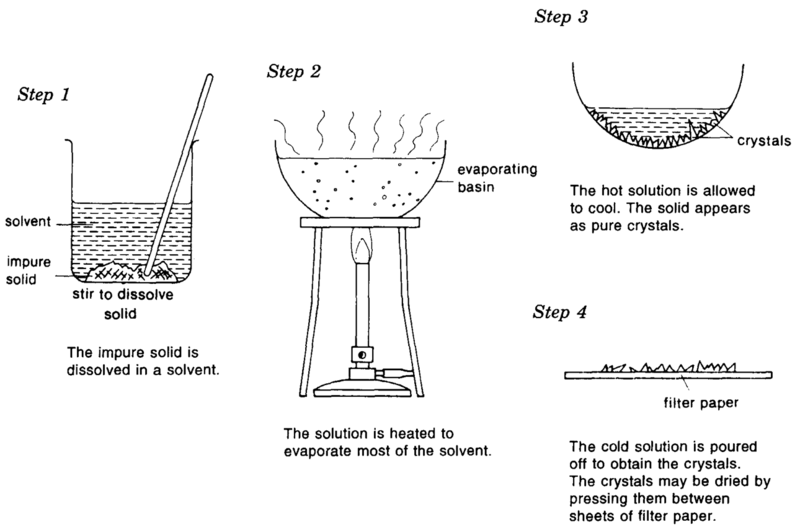
Crystallization
- a solvent is heated up and the solute is then added to the point of saturation; the mixture is left to cool and is left to cool down
- it is primarily used as a purification step
- products can be very pure
- it is primarily used in organic chemistry to purify water soluble substances
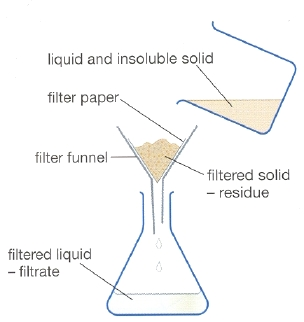
Filtration
- a solution is passed through a porous barrier (filter) which removes any hard solids from a solution
- it is primarily used as a purification step, but can also be used as a separation technique
- permeate (filtrate) is the substances which goes through the filter (usually the goal)
- retenate (residue) is the substance which stays on the filter (usually the waste)
Chromatography
- it is a method of analysing a solution
- an absorber solid (stationary phase) with a sample (a drop of ink for example) is sunk into a solution (mobile phase)
- the solution will rise up due to the capillary action and the individual constituents will slow down based on their polarity
- the drop of ink will spread and show many colors as the different constituents stop at different places of the stationary phase

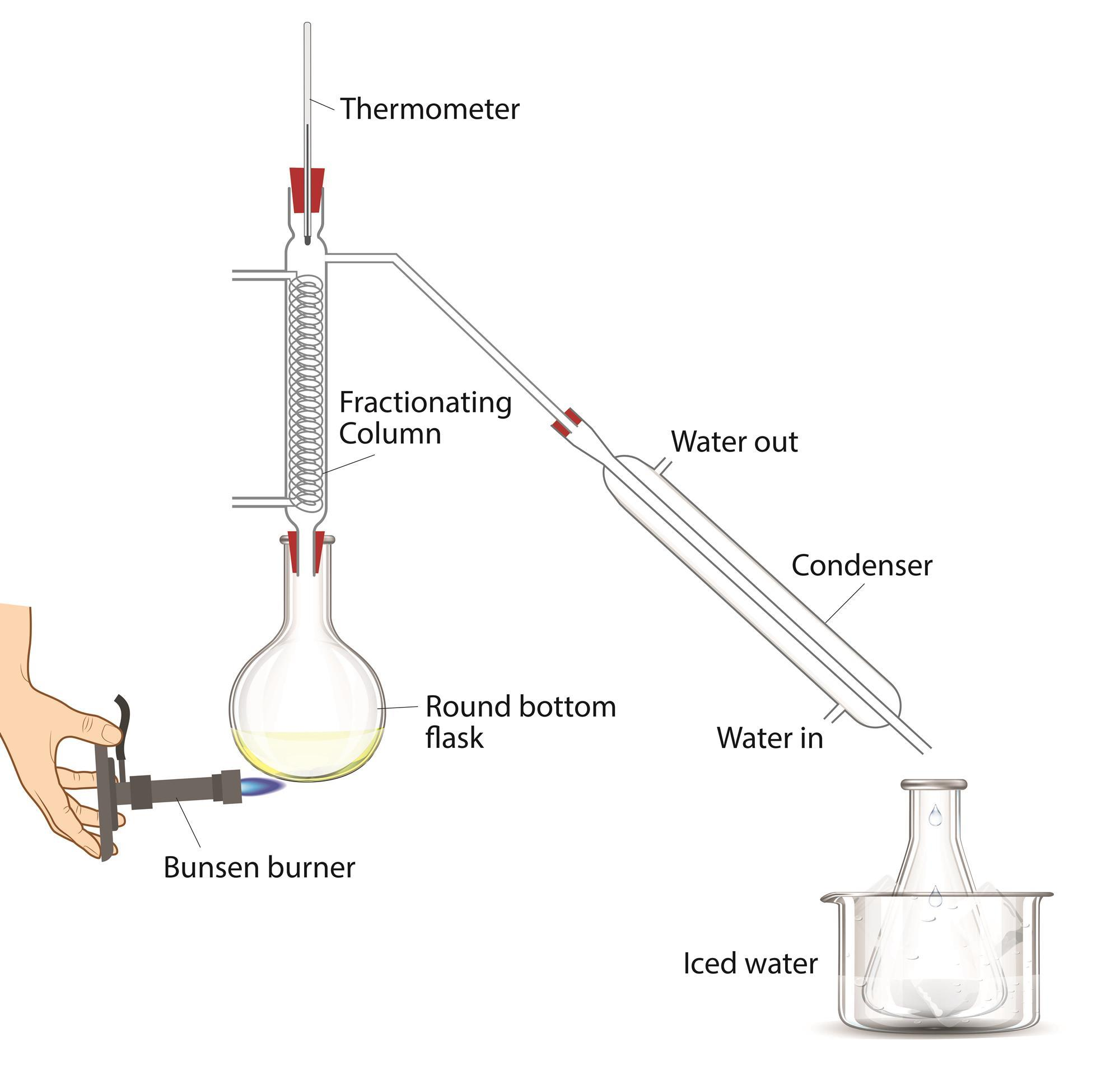
Distillation
- a solution is heated; as the liquids with different boiling points boil, they are liquified again in a condenser and seperated into a different conatiner
- if there are more liquids to be seperated, we call them fractions
- the process is then called fractional distillation
- it is primarily used in petrochemistry and making alcohol
- the process is then called fractional distillation
- the vapor is liquified by cooling it down using cold water in most cases