- chemical bonds are lasting attractions between two atoms
- chemical bonds can be established between two atoms which do not have their valnce electron shell completely filled
- noble gasses (helium, neon and argon primarily) are thus very unreactive and form only a very small number of compounds
- chemical bonds form in three differnet ways:
- electron sharing - covalent bonds
- elecrostatic attraction - ionic bonds
- electron transfer - donor-acceptor bonds
- in all of these cases, the atoms which constitute the bond are as close as possible to the most stable electron configuration of the noble gases
- bonds can be devided into two categories by force:
- strong/primary - the bonds mentioned above and in detail explained below
- weak/secondary - intermolecular forces
Intramolecular force
- intramolecular force is an attraction or any force in general that is formed between two atoms within a molecule
- intramolecular forces include covalent and ionic bonds
Bond length and energy
- bond length is the distance of the two nuclei of the bonding partners
- it is usually meassured in ångströms ($1Å=10^{-10}m$)
- bond energy is measures the bond’s strength and how much energy is needed to destroy it
Bond formation
- as the bonding partners are moving closer together, the potntial energy of the system changes
- when the atoms are distant, the attraction is practically non-existent
- with distance, the energy of the system decreases and thus the system becomes much more stable
- at a point when the attractve force is the same as the rpulsive force of the two atoms, the energy of the system reaches the smallest point and a bond is formed
- the bond length is determined here also
- energy needed to seperate the atoms can be seen
- if the atoms were moved even closer together, the enrgy of the system increases rapidally

- the energy needed to dissociate the bond partners increases as the bond length gets shorter
- triple bonds will thus be much stronger than single bonds
- energy of the bonds with larger atomic readii will be smaller
- bonds get stronger in this order: metalic - non-polar covalent - polar covalent - ionic
Covalent bonds
- covalent bonds are created between atoms when it is most stable fo them to share electrons
- one, two, three or more electron pairs (one electron belonging to each bonding partner) can be shared to form single, double, triple or higher-multiplicity bonds respectively
- the attraction between is stronger with more electron pairs shared
- single, double and triple bonds are the most common in the nature
- higher-multiplicity bonds occure only between lanthanides and actinides
- the bond is formed via an overlap or a strong attraction of orbitals of the bonding partners
$\sigma$ bonds
- $\sigma$ bodning occurs when orbitals overlap
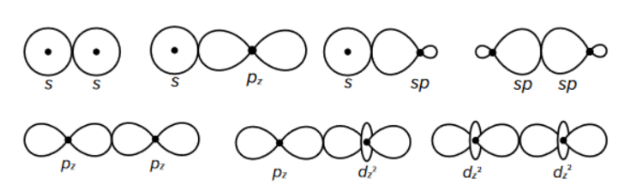
- the orbitals overlap along the internuclear axis (nulceus-nucleus)
- electron density between the two bonding partners is symmetric
- they are the strongest bond between two atoms
$\pi$ bonds
- $\pi$ bonding occurs when orbital overlap on a parallel to the internuclear axis or when they are strongy attracted towards one another
- the electron density is highest above and below the plane of the intermolecular axis
- $\pi$ bonds are formed in double, triple and higher-multiplicity bonds, the single bond is always a $\sigma$ bond
The role of electronegativity
- electronegativity plays a part to the covalent bond as well
- the result of bonding of two atoms of different negativites is that the more electronegative bartner attracts the shared electrons more than the more electropositive bonding partner
- this means that some parts of the molecule are partially charged
Partial charge
- partial charge - $\delta$ - can be either positive - $\delta^+$ - or negative - $\delta^-$
- the bonding partner with higher electronegativity will have a negative partial charge
- the bonding partner with lower electronegativity will have a positive partial charge
- molecules with significant differences of partial charges are called polar
- molecules with insignificant or no partial charges are called non-polar
- if partial charges exist, the bonding partners form an electric dipole
- this plays a massive role in the intermolcular forces between different electric dipoles and it influences the properties of the molecule
- in one molecule, there can be more electric dipoles influencind one another
- the polarity of a molecule depends on where the electric dipoles are and the molecule’s geometry
- if the the strenghts of the dipoles is equal and the dipoles are directly oposite to one another, the molecule is overall non-polar
Differences of electronegativites
- the difference in electronegativities of the bonding partners determines how polar the bond is
- if the difference is low, the bond is less polar
- this is when the difference is less than 0.4
- if the difference is rather high, the bond is more polar
- this is when the difference is in a range from 0.4 to 1.7
- if the differnece is really high, the bond is probably not longer covalent but ionic
- this is when the difference is higher than 1.7
- if the difference is low, the bond is less polar
Ionic bonds
- ionic bonds are created between atoms when it is most stable for them to give up electrons
- typically, ionic bonds are formed between metals and non-metals
- electrons of the bond partners are transfered
- the transfer leads to creation of two oppositely charged particles
- one partner loses one or more valence electrons and a cation is formed
- on partner gains one or more valence electrons and a anion is formed
- the electron configurations of the bodning partners reach as close as possible to the electron configuration of noble gases
- the octet rule states that an atom is most stable when all of its valence orbitals are filled (with eight electrons exactly)
- the duet rule states that it is more stable for an atom to have to atoms in its valnce shell rather that more or less
- ionic compounds have a distinct structure and they form large crystals - for their description, we use the empirical formula
- hence, there are two conditions for an ionic compound to be formed:
- The stability rule - the atom must adhere to the octet or duet rule
- The neutrality rule - the created ionic compound must be neutral and the charges of the ions forming the bond must be balenced out
Structure of ionic solids
Crystaline structure
- the anions and cations of the ionic compound together create a lartger three-dimensional structure - crystaline structure
- the structure is highly regular and forms an ordinal latice
- one ionic compound can have more than one crystline structure
Lattice energy
- the ions together in this crystaline structre are minimazing the lattice energy
- it is the energy of the repulsion and attraction between individual ions
- the attractive forces are at maximum whilst the repulsive forces are at minimum
- it influences many properties of the compound - solubility, hardness, volatility
- ionic compounds are usually very hard but brittle
- they also have very high melting points
- the smaller atomic radii and higher the charge, the higher the lattice energy
- the higher the melting point of an ionic solid, the higher the lattice energy
Valence shells
- electrons in the valence shells are localized (unlike in metalic bonds)
- ionic compounds show very low electric and heat conductivity
- when ionic compounds are heated, the valence shells don’t start to overlap, but the conductivity is still higher, because the ions themselves are able to carry the charge
Coulumb’s law
- the interaction between ions in a crystalline lattice can be described using the Coulumb’s law
- the interaction strength can be approximated using:
$$E=k\cfrac{Q_1Q_2}{d}$$
- where:
- $E$ is the interaction strength
- $k$ is the Coulumb’s constant ($=8.99\cdot{10^{-9}}\ Nm^2C^{-2}$)
- $Q_1$ and $Q_2$ are the charges of the particles
- $d$ is the distance between atomic nuclei
- the overall energy of the interactions is equal to the sum of the energies between all atoms and their combinations in the lattice
- for $NaCl$ that is:
$$ E=E_{Na^+Cl^-}+E_{Na^+Na^+}+E_{Cl^-Cl^-}=\cfrac{-k}{d_{Na^+Cl^-}}+\cfrac{k}{d_{Na^+Na^+}}+\cfrac{k}{d_{Cl^-Cl^-}}$$
Metalic bond
- metalic bond is a special type of bond
- it is created in polymeraic structures of metal crystals
- the orbitals of these metals overlap a lot and all the electrons of these overlapping orbitals are shared in the whole crystal creating the electron gas
Structure of metals and alloys
Pure metals
- metals usually have a much higher number of electrons in the highest enrgy orbitals due to the electrons in d-orbitals and in f-orbitals
- electrons are delocalized in metals and can flow almost freely through the whole lattice
- metalic bond explains many characteristics of metals
- ductility, melleability
- the individual layers of the metalic lattice and theit delocalized electrons are able to easily slide when external force is applied
- heat conductivity, electric conductivity
- the free movement of electrons enables very easy transfer of charge, thus the trasnfer of heat and electricity
- high melting and boiling points
- the attraction of the ions and electrons together is very strong
- ductility, melleability
Alloys
- alloys are mixtures (or combinations) of two or more metals
- they sometimes have other elements added as well
- the metal that composes the majority of the alloy is called the base
- the metal that is added to the base metal is called the dopant
- these combinations of different elements change the properties of pure elements
- the properties of the alloy can be changed by adjusting its composition
- the properties resulting from alloying include strength, hardness, durability and resistence to corrosion
Substitutional alloys
- substitutional alloys are those alloys, where the atoms of the dopant are very similar in size to the atoms of the base metal
- the atoms of the dopant simply substitute the atoms of metal in the lattice
- the final structure can be very similar to that of a pure metal
- examples: bronze, brass
Interstitial alloys
- interstitial alloys are those alloys, where atoms of the dopant are rather different in size to the atoms of the base metal
- the dopant’s atoms are usually smaller
- the atoms of the dopant fill out the empty spaces (intersices) between the metalic cation of the crystaline latice
- the positions of the atoms of the dopant strongly influence the alloy’s properties
Lewis structures
- Lewis strctures (Lewis diagrams or Lewis electron dot diagrams) enable us to write the role of every valence electron in a chemical bond
Lewis symbols
- electrons are represented as dots on the sides of the symbol of an element
- electron pairs are represented as a double dot or more commonly with a line
- typically, it does not matter on which side the unpaired electrons end up, but if there are more than one, the should be on the oposite side
- only valence electrons are included (the orbitals in which they are do not matter)
- ex. Lewis diagram of an oxygen atom - $\cdot\underline{\overline{O}}\cdot$
- the dots can be used to ilustrate ionistion reactions more easily
- ex. Lithium loosing its electron - $Li\cdot\longrightarrow{Li^++e^-}$
Octet rule
- it is most stable for elements to have eight valence electrons in their valence shell
- exceptions are the atoms closer to Helium, where it is most stable for them to have only two electrons in their valence shell (duet rule)
- atoms achive this usually via bonding
- the shared electrons count to the number of electrons in both participants’ valence shells
- these stable atoms can be written using the Lewis diagrams as $|\underline{\overline{X}}|$
Exceptions
- molecules with odd numbers of electrons
- sometimes, it is natural for radicals (substances with one or more unpaired electrons) to occur naturally
- no multiplicity of the bond enables them to have the electron octet
- ex. $NO$ - $\cdot\overline{N}\equiv{O|}$
- over-filled octets
- sometimes, central atoms have more than eight electrons in their valence shell after bonding
- this occurs with elements that can create complex compounds with other types of bonds
- under-filled octets
- sometimes, the central atom’s most stable arrengements are more simple and with less valence electrons than the octet rule suggests
- in these compound, new interesting types of bonding occure
Ionic Lewis dot structures
- Lewis structures of ionic compounds can clearly show where electrons were transfered in a compound
- the ions are usually written in a square parathesis [] with their charges written
- ex. Lewis structure of sodium chloride - $[Na]^+\ [|\overline{\underline{Cl}}|]^-$
Covalent Lewis dot structure
- covalent Lewis structures include also the covalent bonds between elemnts besides their valence electrons
- ex. Lewis structure of carbon dioxide - $\overline{\underline{O}}=C=\overline{\underline{O}}$
Calculating Lewis dot structures
- Writing the formula of the compound and structure analysis
- Writing every known covlent bond 3, Add electron pairs to each atom to reach the octet
- If the number of electron pairs is not in agreement with the octet rule:
- in the case of too many electrons, the free electron pairs are converted to bonds
- in the case of too few electrons, add some to the central atom
Formal charge
- formal charge is the actual charge an atom in a molecule has
- while compounds are usually most stable in neutral states, individual atoms can be charged in the molecule
- the overall charge of the molecule stays neutral
- while compounds are usually most stable in neutral states, individual atoms can be charged in the molecule
Calculating formal charge
- formal charge can be calculated using a Lewis diagram
- it is calculated as follows: $$Q_f=n_v-(n_n+\frac{1}{2}n_b)$$
- where:
- $Q_f$ is the formal charge
- $n_v$ is the number of valence electrons (in a free atom)
- $n_n$ is the number of non-bonding electrons
- $n_b$ is the number of bonding electrons
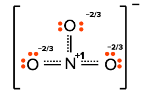
Resonant structures
- resonance structures are a set of at least two Lewis diagrams which collectively describe the electronic bonding in a molecule
- this also accounts for fractional bonding
- they can also be used to describe a system of delocalized electrons
- they are used whenever we do not know with certainty which lewis structre is correct
- the propabilites of double bonds for example can be the same in two different cases
- the existance of a resonant structure has been proven by meassuring the bond length of a molecule with multiple possible lewis diagrams
- the bond lengths were quite far off the theoretical value
- ex. Resonance structure of $NO_3^-$
Structure of molecules
- the resonant structures, Lewis diagrmas and formulae are representing a two dimensional molecule, VSEPR and the theory of hybridization helps us explain the three dimensional structure
- bond length is the straight distance between the nuclei of two bonded atoms
- it is usually measured in ånsgtröms or picometers
- $1\ Å=10^{-10}m$
- $1\ pm=10^{-12}m$
- it is usually measured in ånsgtröms or picometers
- bond angle is the angle between any two chosen bonds which intersect with one another in one common atom
- it is usually measured in degrees or radians
Hybridization
- hybridization is a mathematical concept which describes the using of atomic orbitals to form new hybridized orbitals of the same energy
- the new orbitals have different energies nad shapes from the individual atomic orbitals
- these new orbitals are more sutable for the bonds in the molecule
- valence orbitals are most likely to hybridize
- d-orbitals can also hybridize
- the number of new hybridized orbitals is equal to the number of originally involved atomic orbitals
- hybridization occurs when the participating orbitals have more or less similar energy and are suitably symmetric
- the type of hybridization depends on the atomic orbitals involved
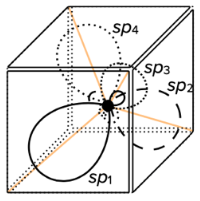
sp hybridization
- the hybridization of one s-orbital and one p-orbital results in the creation of two hybridized sp-orbitals of the same energy
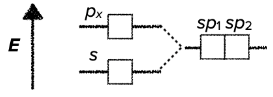
- the shape of the sp-orbitals is different
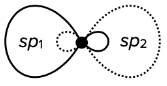
- sp hybridization results in linear geometrical structure
- it usually involves two $\sigma$ bonds
- the bond angles around the central atom are 180°
sp$^2$ hybridization
- the hybridization of one s-orbital and two p orbitals results in the creation of three hybridized sp$^2$-orbitals
- sp$^2$ hybridization results in trigonal planar geometrical structure
- it usually involves three $\sigma$ bonds
- the bond angles around the central atom are 120°
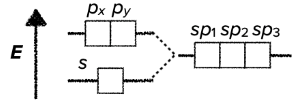
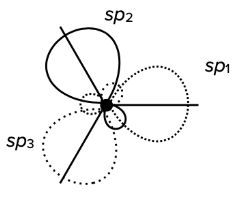
sp$^3$ hybridization
- the hybridization of one s-orbital and three p-orbitals results in the creation of four hybridized sp$^3$-orbitals
- sp$^3$ hybridization results in tetrahedral geometrical structure
- it usually involves four $\sigma$ bonds
- the bond angles around the central atom are approximatelly 109.5°
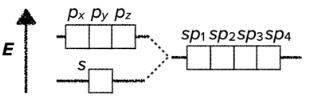
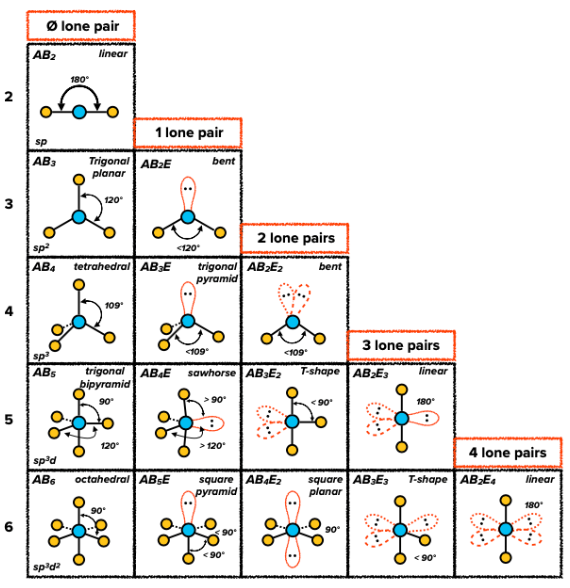
VSEPR - Valence Shell Electron Pair Repulsion
- some molecules do not follow the standard theory of hybridization because of their free electron pairs acting repulsing the other electrons of the bonds
- the bond angles are a little deformed as a result of the repulsion
- VSEPR predicts the shapes of molecules based on an order of repulsion
- lone pairs occupy the least ammount of space and have the highest electron density
- bonding pairs occupy the most ammount of space and have the lowest electron density
- higher multiplicity bonds occupy less space than lower multiplicity bonds and have higher electron density
- oder of repulsion (from strongest to weakest)
- lone pair - lone pair
- lone pair - bonding pair
- bonding pair - bonding pair
Predicting the electorn pair geometry
- Drawing the Lewis diagram
- Identify the hybridization based on the number of $\sigma$ bonds
- Place the lone electron pairs of the central atom accordng to the order of repulsion
VSEPR Shapes